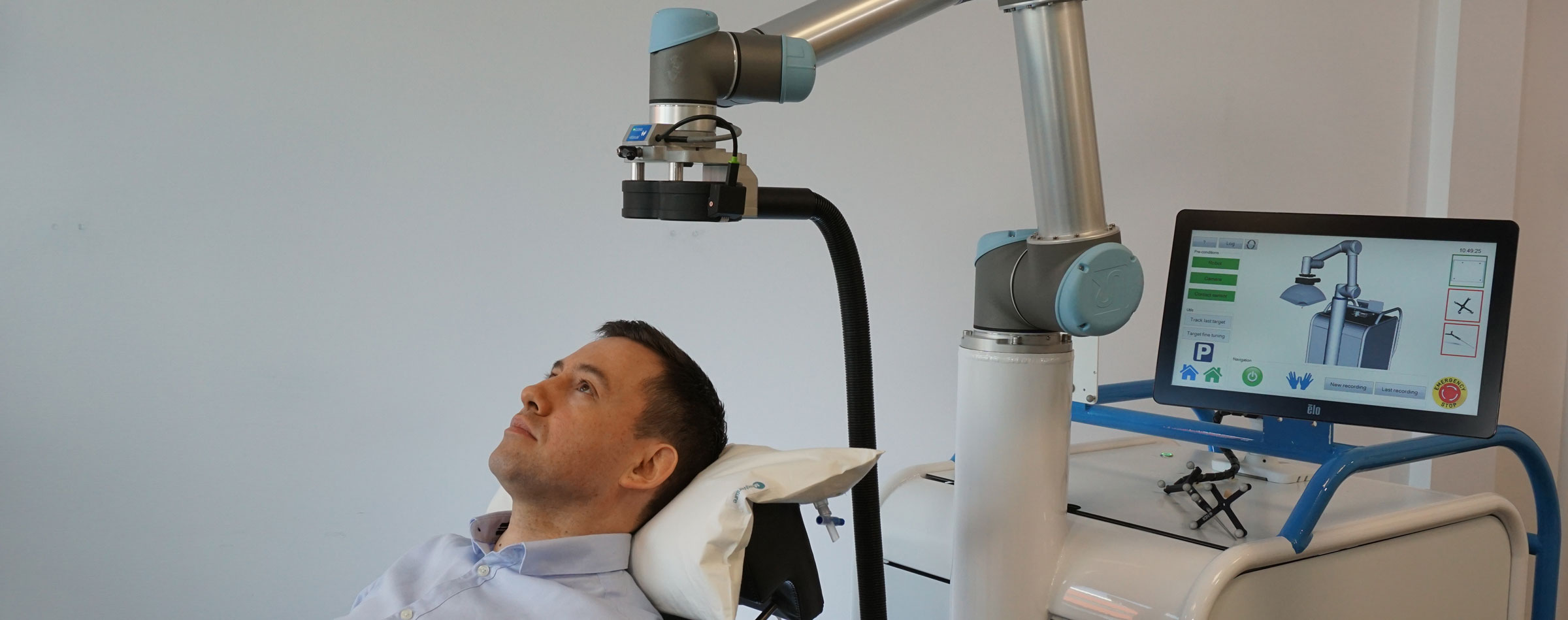
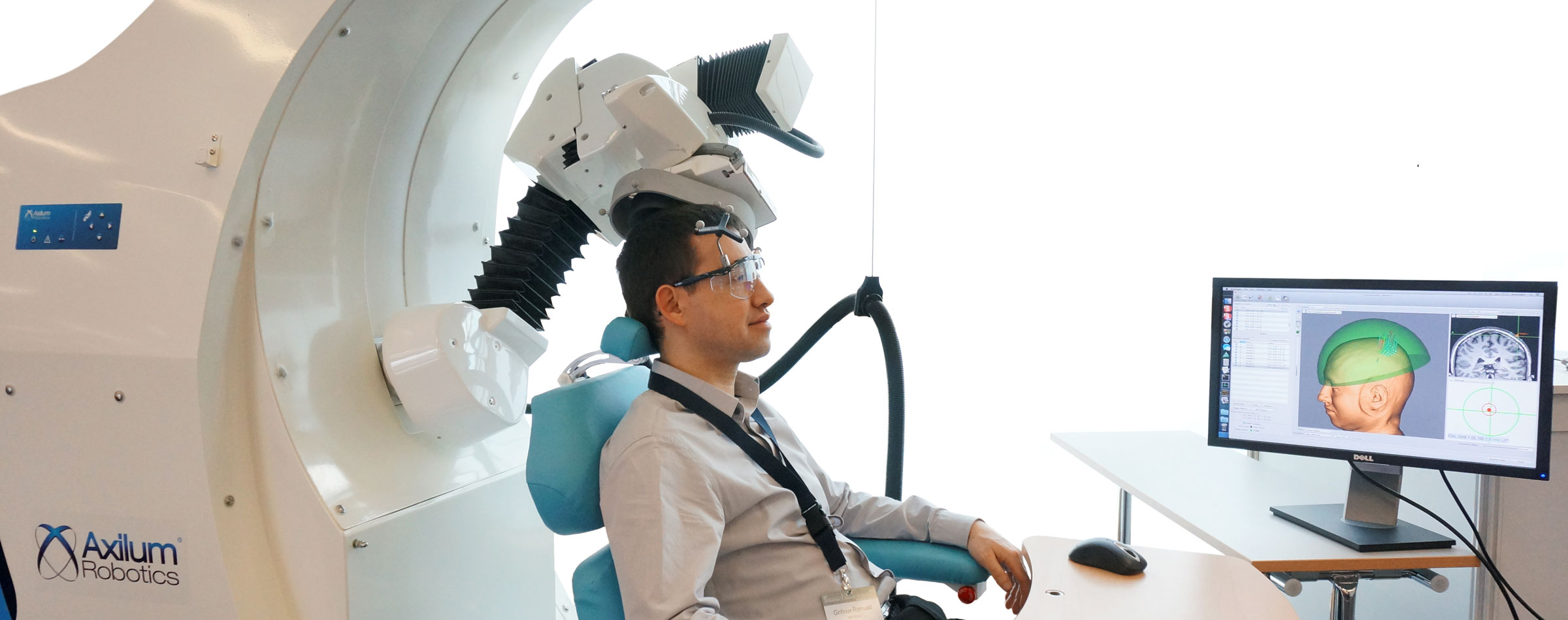
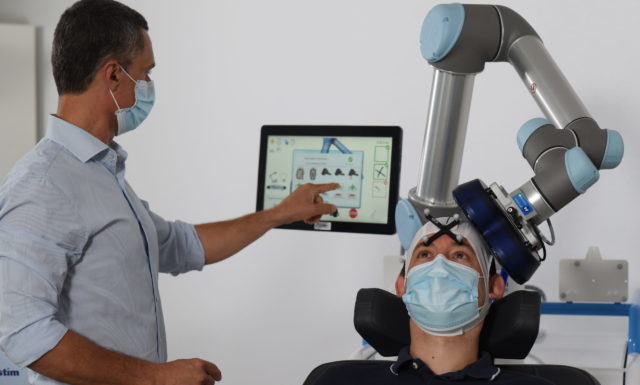
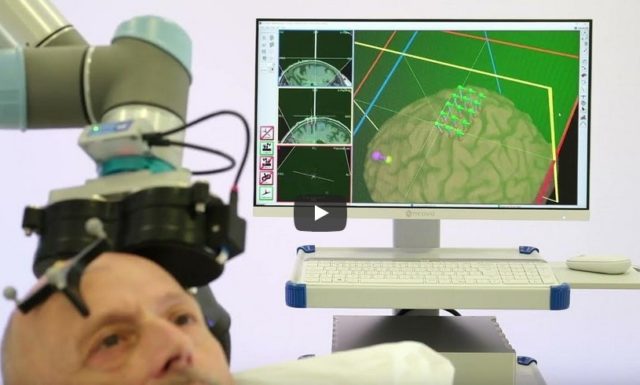
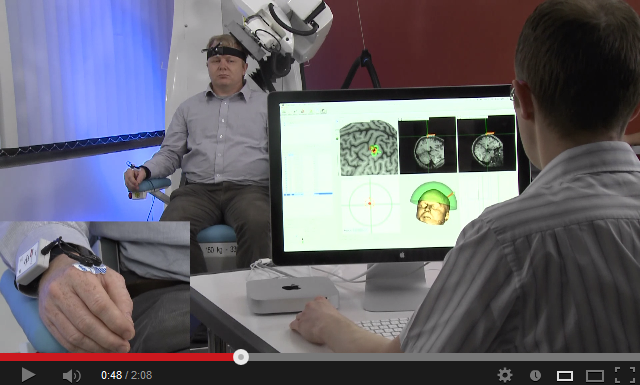
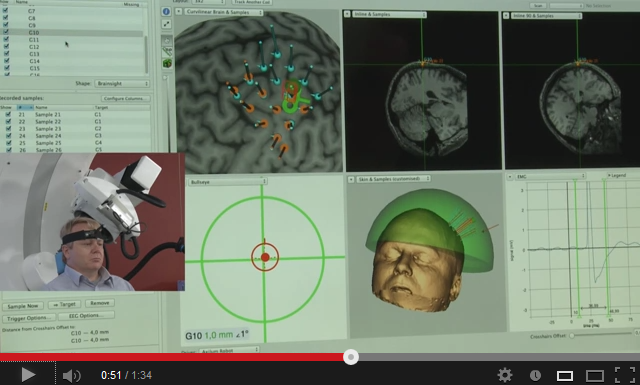
After having developed and commercialized TMS-Robot, the first robot specifically designed for Transcranial Magnetic Stimulation (TMS), Axilum Robotics enlarges its range of robotic solutions for TMS with TMS-Cobot, intergrating collaborative robotic or « cobot » technology.
TMS-Robot and TMS-Cobot allow to automate this non-invasive, painless brain stimulation technique, usually implemented manually, with a high level of safety and with improved accuracy and repeatability.
With its partners, Axilum Robotics gives a unique opportunity to healthcare professionals and researchers to improve the execution of their TMS procedures, using different robotic solutions, while saving the operator from a repetitive and painful task.
Axilum Robotics TMS-Cobot is a CE marked medical device, has FDA 510(k) clearance in the USA, TGA approval in Australia and HSA approval in Singapore.
Axilum Robotics TMS-Robot is a device available for medical or scientific investigation purposes only.